-
- 2023.11.8
Ai-BrainScience Secures SaMD Approval in Japan, MIREVO® as Dementia Screening Tool
Ai-BrainScience Inc (Head Office: Osaka, CEO: Kentaro Takamura Ph.D., “the company/we” hereof) is thrilled to announce that the company received a marketing authorization from the Ministry of Health, Labor and Welfare in Japan for MIREVO®. This neuropsychological assessment application stands as a first in Japan. We intend for MIREVO® to be covered by Japanese National Health Insurance. In a strategic move, the company has partnered with Otsuka Pharmaceutical Inc., (Head Office: Tokyo, CEO: Makoto Inoue) who will act as the exclusive sales distributor for MIREVO® in the Japanese market.
MIREVO® is a Software as Medical Device (SaMD) designed for use with the Apple iPad to install. Based on a patient’s gaze patterns on the iPad screen, it provides 3-minute automatic assessment. This approach eliminates dependence on physician’s expertise, facilitating a more objective and quantitative assessment.
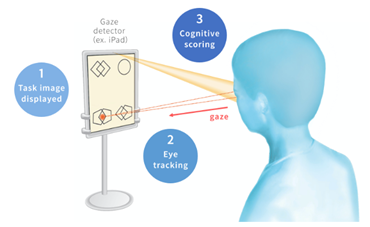
The company completed a clinical trial in Japan whose target population was patients clinically diagnosed with dementia and subjects with cognitively healthy controls and MCI*¹. This trial verified a positively high correlation in total score between this application and MMSE*² as the primary endpoint. Also, the trial confirmed that the examiner’s mental stress was reduced compared with MMSE as the secondary endpoint.
With recommendations in the international clinical practice guidelines*³*⁴, MMSE is widely used as dementia screening tool for diagnostic purpose. MMSE assessment, however, often stretching up to 20 minutes*⁵, requires a high level of expertise, and often results in about 70% of patients reportedly experiencing a high degree of mental stress*⁶. The company is confident that MIREVO® will address such challenges, contributing to earlier detection of cognitive decline.
The genesis of the eye-tracking cognitive assessment tool, MIREVO®, can be traced back to the clinical research from Osaka University’s Geriatric Medicine Graduate School. The commercial development originates from START Project hosted by Japan Science and Technology Agency (JST), start-up support program for academic high-potential technology seeds.
*1:Mild Cognitive Impairment
*2:Mini Mental State Examination (widely-used neuropsychological assessment tool for dementia screening)
*3 Clinical Practice Guideline for Dementia 2017, Japanese Society of Neurology
*4:APA GUIDELINES for the Evaluation of Dementia and Age-Related Cognitive Change
*5:Haubois et al. BMC Geriatrics 2011, 11:59
*6:Self-Reported Distress After Cognitive Testing In Patients With Alzheimer’s Disease, August 2008
Product Overview
Brand Name MIREVO® Generic Name Neuropsychological Assessment Application Program Approval No. 30500BZX00235000 Intended for Use Neuropsychological Assessment based on patients’ gaze patterns, assisting dementia diagnosis Approval Date 5th October 2023 Device for Use iPad Pro (12.9 inch) <Contact>
Ai-BrainScience Inc.
TEL: +81-3-6272-6744
e-Mail:info@ai-brainscience.co.jp


